Become a StemSpine® Provider and Join
the Regenerative Medicine Revolution

Exclusivity Within Region

On-Site Training

Marketing & Sales Support
StemSpine® is Backed by Science
StemSpine® is a patented stem cell based treatment for treatment of chronic lower back pain. It is provided by Creative Medical Technology Holdings, Inc., a commercial stage biotechnology company committed to improving patient lives in the areas of urology, neurology, and orthopedics utilizing stem cells. StemSpine® is the direct result of research and collaboration between some of the preeminent scientists and physicians in the world who specialize in orthopedics/neurology and stem cell therapy.
Developed by Leaders
StemSpine® has the pedigree of being developed by leaders in the field of regenerative medicine and offers a drug free alternative to patients suffering with Chronic Lower Back Pain.
Compliance Focused
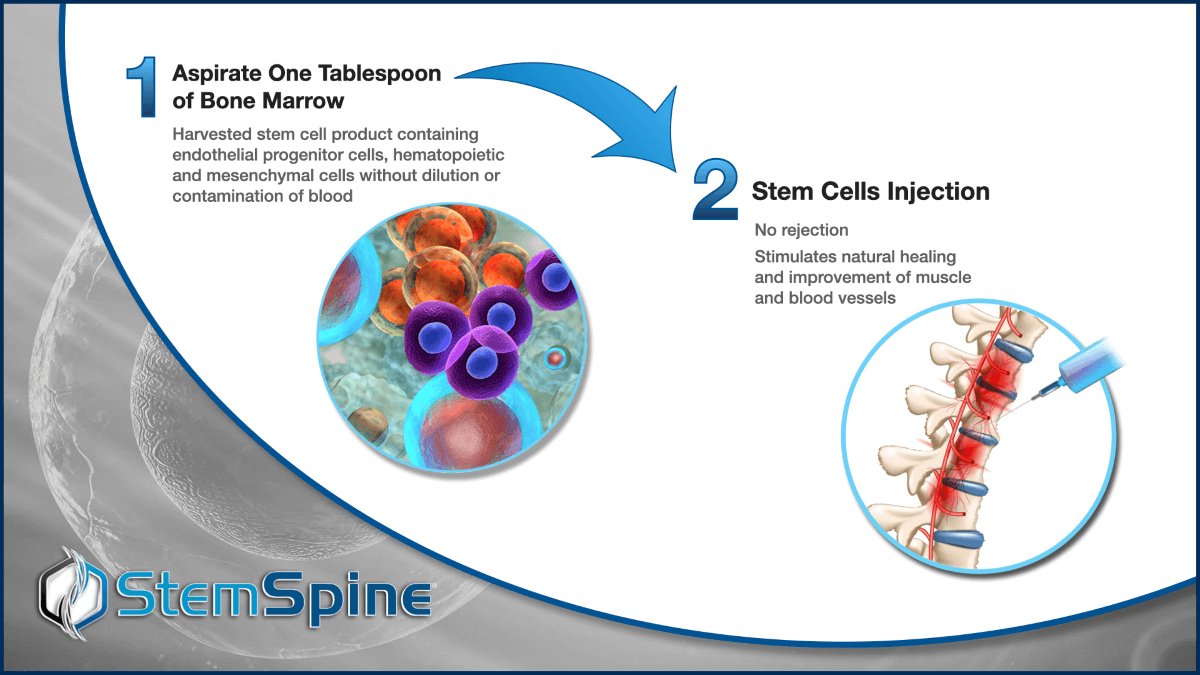
45 Minute Outpatient Procedure
Performed Under Local Anesthetic Using Closed System Device that Does Not Require Centrifuge / Filters and is FDA Compliant Under 21 CFR 127.15(b)*
Performed Under Local Anesthetic Using Closed System Device that Does Not Require Centrifuge / Filters and is FDA Compliant Under 21 CFR 127.15(b)*
* Program has not been evaluated by the FDA
Become a Provider
Contact us to Get Started


