
What is StemSpine®
StemSpine® is a patented stem cell procedure for treatment of Chronic Lower Back Pain (CLBP) offered by Creative Medical Technology Holdings, Inc. StemSpine® is performed using FDA-approved equipment by a licensed physician that provides a safe, effective, drug free solution to treat CLBP.
How StemSpine® Works
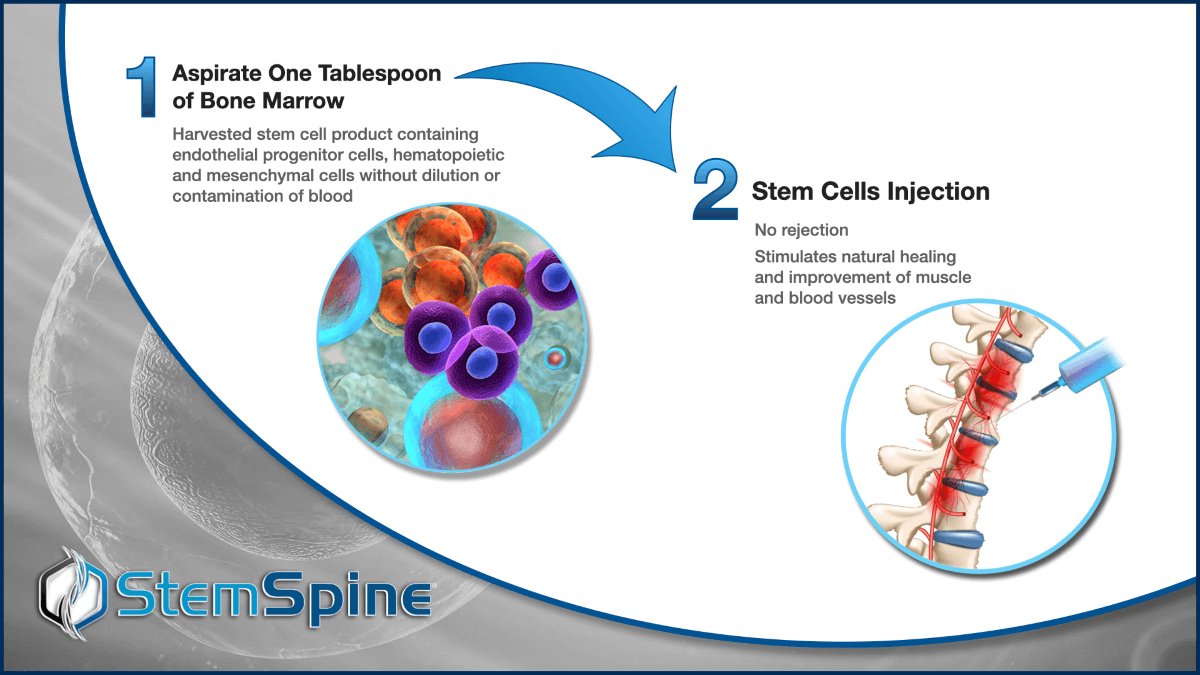
- Injected bone marrow aspirate increases perfusion of area associated with disc pain
- Increased perfusion allows for clearance of toxic metabolites
- Increased perfusion triggers endogenous tissue repair and cellular recovery
- This results in healing of the surrounding muscle and disc space that reduces pain and improves mobility
* Program has not been evaluated by the FDA
About Chronic Lower Back Pain
- Chronic Lower Back Pain (CLBP) is the leading source of disability in people under 45 years of age
- More than 80% of the US population with experience an episode of LBP at some point during their lives, some of which will not recover and develop CLBP
- An estimated 149 million days of work per year are lost because of CLBP
- CLBP is costly, with total costs estimated to be between $100 and $200 billion annually
- There are currently limited non-surgical treatment options for CLBP, and there is limited data to demonstrate that patients who undergo surgical fusion have better outcomes than those who pursue conservative treatments
Conclusion
Chronic Lower Back Pain is a major healthcare crisis and StemSpine® is a non-surgical approach
targeting the root cause of this debilitating disease.
targeting the root cause of this debilitating disease.
The StemSpine® Difference

Treatment
Non-surgical treatment option with no dependence on pain medications

Approach
Utilizing a patient's own stem cells in a regenerative approach

Recovery
Minimal down time away from work and family
Contact Us
Learn more about StemSpine® today.

